Motivation
Superhard materials are crucial to established technologies such as cutting, polishing, drilling, mining, and grinding, both as bulk materials and as wear-resistant coatings. The steadily increasing superhard materials market revenue reached 4.5 billion USD in 2017, with an estimated growth rate of 5.5% through 2025. The dominant material in this industry is diamond, which is the hardest known material; despite its many superlative properties, its applications are limited by low toughness, tendency toward oxidization, reactivity with ferrous metals, and current production cost. Thus, new superhard materials are needed to support numerous emerging technologies, including materials with performance that exceed diamond and other conventional superhard materials. Moreover, new technologies require advanced superhard and high-strength materials with additional functionalities, for example, for enhanced performance in extreme environments. To this end, recent work has shown the promise of a new generation of such materials that are also hard superconductors, quantum information storage materials, novel topological systems, photonic and optical communication materials, photovoltaic components, ultra-low dielectric response materials, superhard thermoelectrics, nuclear power components, and high-energy density materials.
These efforts have been driven by recent theoretical advances in first-principles and machine-learning based methods combined with the development of new experimental methods, including the creation of novel phases at high pressure-temperature (P-T) conditions and techniques for synthesizing metastable phases under ambient conditions. A number of combinations of light elements such as boron nitrides, carbon nitrides, boron carbides, boron oxides, ternary B-C-N/B-C-O/B-N-O compounds or carbon polymorphs have been explored theoretically and experimentally. Indeed some of these materials are predicted to possess physical properties that are superior to diamond. Similarly, compounds of light elements (C, B, N, O) with transition metals are now of great interest.
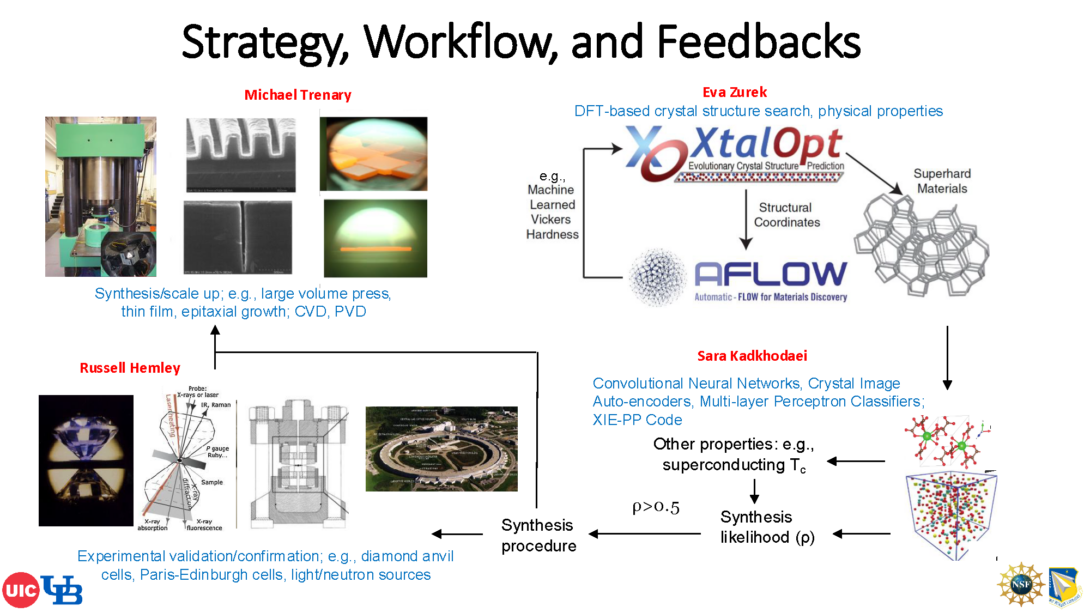
We have assembled a unique team with complementary strengths to address the challenge of creating next generation superhard materials, including those with novel functionalities. Russell Hemley (PI) is an expert in high-pressure experiments, superhard materials and diamond growth, and first-principles calculations. Eva Zurek (co-PI) has pioneered simulations for predicting new superhard materials, and has developed a variety of open-source codes for materials discovery and used them to predict numerous novel materials. Sara Kadkhodaei (co-PI) has expertise in both theoretical studies of thermodynamics and kinetics of solids and deep-learning methods, and Michael Trenary (co-PI) is a surface scientist with considerable experience on studies of borides and other boron-rich solids. Working together and with our collaborators, this project will therefore advance the goals of the Materials Genome Initiative.
Approach
We focus on intrinsic material hardness and other measures of strength derived from elastic properties as described below, but also novel ultrahard films and heterostructures. The light elements such as B, C, N, and O that typically comprise known superhard materials can form short and strong covalent bonds, and may have complex potential energy surfaces with numerous low lying local minima. Because the main atomic constituents of such superhard materials have similar masses, their crystal structures can be difficult to determine. As a result, theoretical studies have been instrumental in uncovering the structures of superhard compounds, including the M-carbon phase that was synthesized by the PI’s group by cold compression of graphite. Moreover, because many superhard materials are metastable at ambient conditions, and the synthesis procedure can have an impact on the product that is made, a synergistic feedback loop between theory and experiment is required for their rational design and discovery.
Two different computational approaches have been used to pinpoint superhard materials to date. The first couples density functional theory (DFT) calculations with crystal structure prediction (CSP) methods and microscopic hardness models. This approach can be used to predict structures that are unknown, but the models used to estimate hardness require ill-defined quantities that can yield unphysical results. In the second approach the properties stored in large materials databases are used to train machine learning (ML) models of the elastic moduli, which are required as input in macroscopic hardness models. This approach has excellent predictive power, and is extremely fast, but it can only be used to screen previously synthesized materials. Co-PI Zurek has recently combined these two techniques by merging the XtalOpt evolutionary algorithm (EA) for CSP with an ML model trained on the AFLOW materials database. The manuscript outlining the new methodology was recently published, and highlighted in the media. Popular Mechanics explained that “the team… used artificial intelligence to identify 43 previously unknown forms of carbon that are thought to be stable and superhard.” This novel CSP method will be harnessed towards superhard materials discovery. Advanced statistical learning methods will be utilized to predict the synthesis likelihood and synthesis pathways for these hypothetical phases, introducing new criteria to guide the computation-enabled discovery of new useful materials. Two promising ways to synthesize novel superhard materials, especially those with additional functionality, are (1) high pressure-temperature (P-T) methods, and (2) metastable growth and surface deposition techniques. Thus, the main goals of this project are to:
- Employ Zurek’s new DFT+ML CSP method to pinpoint superhard binary and ternary phases containing B, C, and/or N, as well as transition metal carbides, borides and nitrides, that are currently unknown. Use DFT to calculate the physical properties of these functional materials.
- Develop ML-based models to predict the synthesizability of promising phases, and shed insights on synthesis strategy. Perform DFT calculations to study the kinetics of phase transitions, or chemical reactions, that can occur during synthesis and impact stability.
- Use state-of-the art high pressure and metastable growth techniques to search for, synthesize, and characterize phases targeted by theory. Carry out first-principles calculations to interrogate phase and kinetic stability as a function of pressure, temperature and surface growth.
- Employ a feedback loop between experiment and theory to characterize synthesized phases, and improve synthesis protocols, including ways to recover high-pressure phases to ambient conditions.
History shows us that ingenious ways can be found to make technologically important compounds. Consider diamond, which is less stable than graphite at 1 atm and 298 K, forming within the Earth at 4.5-6 GPa. Because the barriers to decomposition are so high (due to the strong C-C sp3-bonds), diamond is metastable at 1 atm. In the 1950’s researchers reported methods to synthesize diamond using large presses to attain pressures of ~5 GPa at C. Diamond can be made using low pressure techniques as well, namely via chemical vapor deposition (CVD). In 2002, PI Hemley developed a high growth rate technique that enabled the production of large, high-quality single-crystal CVD diamond leading to 18 patents. Now polycrystalline and single crystal diamond are grown routinely via CVD techniques. This is one example where the production of materials initially synthesized under high pressure has been commercialized, but more importantly it sets the stage for the creation of new advanced diamond-based materials. Extending these low-pressure synthesis approaches to other systems, including scale up for other applications, remains an experimental challenge. To address this challenge, we will use a synergistic computational/experimental approach to discover, synthesize, and recover next generation materials with superior hardness and other high strength properties, with a focus on novel functionalities required for future technologies. The project will also engage experts at Department of Defense (AFRL) and several Department of Energy laboratories.